The pH for 0.0850 M solution of C6H:CH2COOH is 2.68. Determine the value of Ka for CsHsCH2COOH. PREV 2 Based on your ICE table and definition of Ka, set up the expression for Ka and then evaluate it. Do not combine or simplify terms. Ka 5 RESET [0] [0.0850] [2.68] [2.1 x 10) [0.0829] [0.428] [4.79 x 10-"1 1.9 x 10* 40 0.025 5.3 x 105 II
The pH for 0.0850 M solution of C6H:CH2COOH is 2.68. Determine the value of Ka for CsHsCH2COOH. PREV 2 Based on your ICE table and definition of Ka, set up the expression for Ka and then evaluate it. Do not combine or simplify terms. Ka 5 RESET [0] [0.0850] [2.68] [2.1 x 10) [0.0829] [0.428] [4.79 x 10-"1 1.9 x 10* 40 0.025 5.3 x 105 II
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter16: Principles Of Chemical Reactivity: The Chemistry Of Acids And Bases
Section16.2: Water And Ph Scale
Problem 3RC: If the pH of a solution containing the strong base Sr(OH)2 is 10.46 at 25C, what is the...
Related questions
Question
![The pH for 0.0850 M solution of C6H:CH2COOH is 2.68. Determine the value
of Ka for CsHsCH2COOH.
PREV
2
Based on your ICE table and definition of Ka, set up the expression for Ka and then evaluate
it. Do not combine or simplify terms.
Ka
5 RESET
[0]
[0.0850]
[2.68]
[2.1 x 10)
[0.0829]
[0.428]
[4.79 x 10-"1
1.9 x 10*
40
0.025
5.3 x 105
II](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F108a0acc-c86a-4fb9-81b6-4d3403f04067%2F796182e1-9e6e-4dbb-bfe7-94d3a6b08131%2F6ivffkj_processed.png&w=3840&q=75)
Transcribed Image Text:The pH for 0.0850 M solution of C6H:CH2COOH is 2.68. Determine the value
of Ka for CsHsCH2COOH.
PREV
2
Based on your ICE table and definition of Ka, set up the expression for Ka and then evaluate
it. Do not combine or simplify terms.
Ka
5 RESET
[0]
[0.0850]
[2.68]
[2.1 x 10)
[0.0829]
[0.428]
[4.79 x 10-"1
1.9 x 10*
40
0.025
5.3 x 105
II

Transcribed Image Text:The pH for 0.0850 M solution of C&HSCH:COOH is 2.68. Determine the value
of Ka for C&HSCH:COOH.
1
2
NEXT >
Based on the given values, fill in the ICE table to determine concentrations of all reactants
and products.
C&H:CH:COOH(a_
q)
CoHsCH:COO-(aq
H:O(1)
H:O*(aq)
Initial (M)
Change (M)
Equilibrium (M)
5 RESET
0.0850
2.68
-2.68
2.1 x 10
-2.1 x 10
0.0829
-0.0829
0.428
-0.428
1L
Expert Solution
Step 1
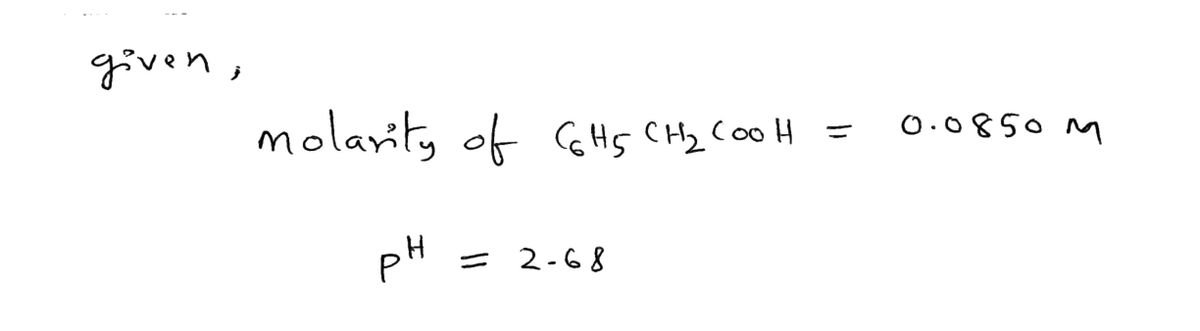
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning